



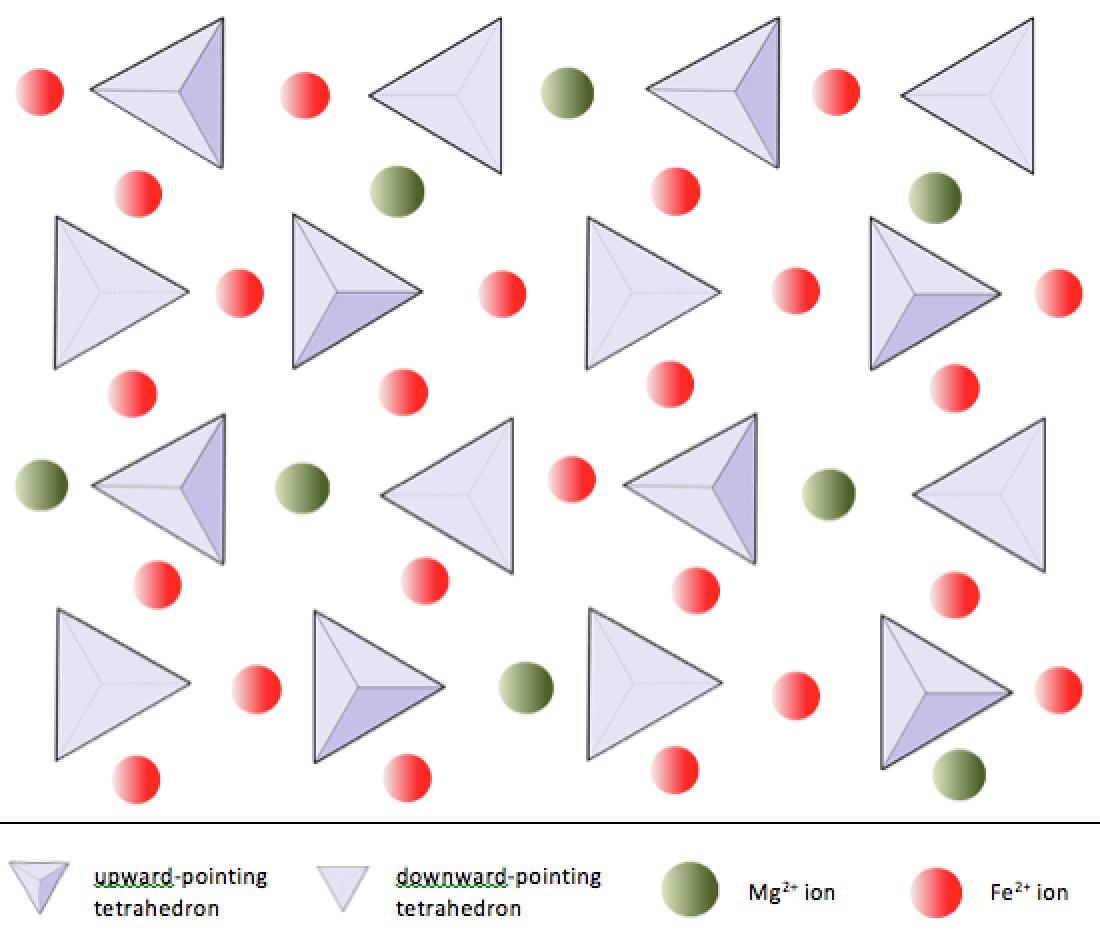
Silica tetrahedra can be arranged and linked together in a variety of ways, from single units to complex frameworks (Table 3.1). The simplest silicate structure, that of the mineral olivine, is composed of isolated tetrahedra bonded to iron and/or magnesium ions. In olivine, the −4 charge of each silica tetrahedron is balanced by two divalent (i.e., +2) iron or magnesium cations. Olivine can be either Mg2SiO4 or Fe2SiO4, or some combination of the two (Mg,Fe)2SiO4.
Recall that for non-silicate minerals, we classified minerals into groups according to their anion or anionic group. For silicate minerals, we group minerals based on their silicate structure into groups called: isolated, pair, ring, single chain, double chain, sheet, and framework silicates. In this course, we will focus on just the isolated, single chain, double chain, sheet, and framework silicates.
| Tetrahedron Configuration Picture | Tetrahedron Configuration Name | Example Minerals |
|---|---|---|
| Isolated (nesosilicates) | Olivine, garnet, zircon, kyanite | |
| Pairs (sorosilicates) | Epidote, zoisite | |
 | Rings (cyclosilicates) | Tourmaline |
 | Single chains (inosilicates) | Pyroxenes, wollastonite |
 | Double chains (inosilicates) | Amphiboles |
 | Sheets (phyllosilicates) | Micas, clay minerals, serpentine, chlorite |
| 3-dimensional structure | Framework (tectosilicates) | Feldspars, quartz, zeolite |
In olivine, unlike most other silicate minerals, the silica tetrahedra are not bonded to each other. Instead they are bonded to the iron and/or magnesium ions, in the configuration shown on Figure 3.1.1.
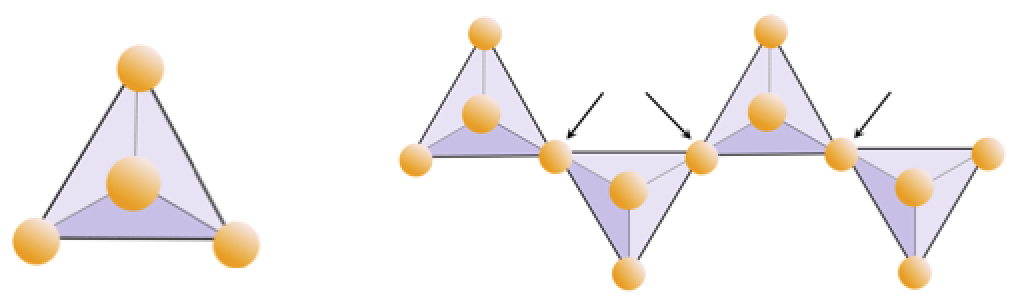
The structure of the single-chain silicate pyroxene is shown on Figures 3.1.2 and 3.1.3. In pyroxene, silica tetrahedra are linked together in a single chain, where one oxygen ion from each tetrahedron is shared with the adjacent tetrahedron, hence there are fewer oxygens in the structure. The result is that the oxygen-to-silicon ratio is lower than in olivine (3:1 instead of 4:1), and the net charge per silicon atom is less (−2 instead of −4). Therefore, fewer cations are necessary to balance that charge. Pyroxene compositions are of the type MgSiO3, FeSiO3, and CaSiO3, or some combination of these, written as (Mg,Fe,Ca)SiO3, where the elements in the brackets can be present in any proportion.
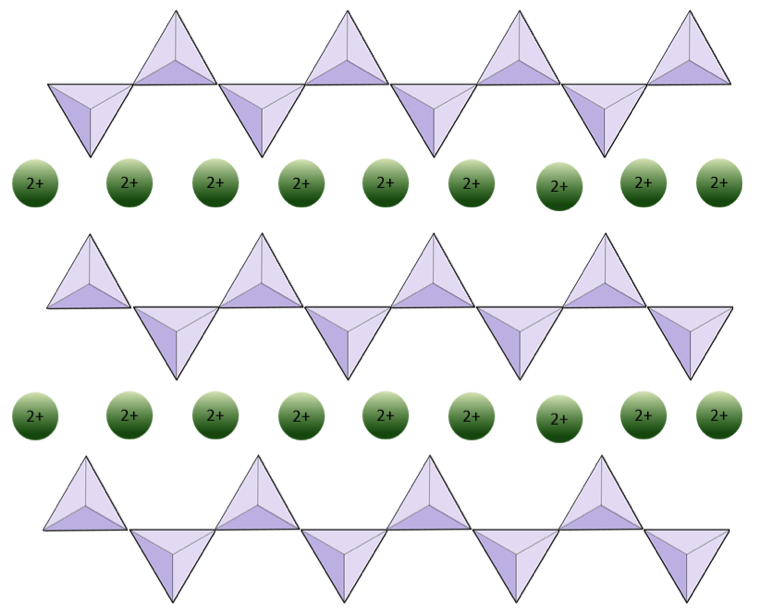
In amphibole structures, the silica tetrahedra are linked in a double chain that has an oxygen-to-silicon ratio lower than that of pyroxene, and hence still fewer cations are necessary to balance the charge. Amphibole is even more permissive than pyroxene and its compositions can be very complex. Hornblende, for example, can include sodium, potassium, calcium, magnesium, iron, aluminum, silicon, oxygen, fluorine, and the hydroxyl ion (OH−).
In mica minerals, the silica tetrahedra are arranged in continuous sheets. There is even more sharing of oxygens between adjacent tetrahedra and hence fewer cations are needed to balance the charge of the silica-tetrahedra structure in sheet silicate minerals. Bonding between sheets is relatively weak, and this accounts for the well-developed one-directional cleavage in micas. Biotite mica can have iron and/or magnesium in it and that makes it a ferromagnesian silicate mineral (like olivine, pyroxene, and amphibole). In muscovite mica, the only cations present are aluminum and potassium; hence it is a non-ferromagnesian silicate mineral.
Apart from muscovite and biotite, there are many other sheet silicates (a.k.a. phyllosilicates), many of which exist as clay-sized fragments (i.e., less than 0.004 millimetres). These include the clay minerals like kaolinite. Although they are difficult to study because of their very small size, they are extremely important components of rocks and especially of soils.
Silica tetrahedra are bonded in three-dimensional frameworks in both the feldspars and quartz. These are non-ferromagnesian minerals—they don’t contain any iron or magnesium. In addition to silica tetrahedra, feldspars include the cations aluminum, potassium, sodium, and calcium in various combinations. Quartz contains only silica tetrahedra.
The three main feldspar minerals are potassium feldspar, (a.k.a. K-feldspar or K-spar) and two types of plagioclase feldspar: albite (sodium only) and anorthite (calcium only). As is the case for iron and magnesium in olivine, there is a continuous range of compositions (solid solution series) between albite and anorthite in plagioclase (i.e., intermediate compositions between NaAlSi3O8 and CaAl2Si3O8 can exist).
In quartz (SiO2), the silica tetrahedra are bonded in a “perfect” three-dimensional framework. Since in every silica tetrahedron one silicon cation has a +4 charge and the two oxygen anions each have a −2 charge, the charge is balanced. There is no need for aluminum or any of the other cations such as sodium or potassium. The hardness and lack of cleavage in quartz result from the strong bonds characteristic of the silica tetrahedron.
Family names versus mineral names
The names “pyroxene”, “amphibole”, “mica”, and “feldspar” can be confusing at first, as these are technically names of mineral “families” and not names of a specific mineral. Minerals within the same family tend to share common structures, but each individual mineral is distinguished by its chemical formula. In the examples below the mineral names are bolded.
Practice Exercise 3.1 Ferromagnesian silicates?
Silicate minerals are classified as being either ferromagnesian or non-ferromagnesian depending on whether or not they have iron (Fe) and/or magnesium (Mg) in their formula. A number of minerals and their formulas are listed below. For each one, indicate whether or not it is a ferromagnesian silicate.
| Mineral | Formula | Ferromagnesian silicate? |
| olivine | (Mg,Fe)2SiO4 | . |
| pyrite | FeS2 | . |
| plagioclase feldspar | CaAl2Si2O8 | . |
| pyroxene | MgSiO3 | . |
| hematite | Fe2O3 | . |
| orthoclase feldspar | KAlSi3O8 | . |
| quartz | SiO2 | . |